A Guide to Preimplantation Genetic Testing (PGT) for Sickle Cell
Introduction: Simplifying PGT for the Public
We receive many inquiries about genetic diagnosis. Therefore, I will provide a simplified guide to Preimplantation Genetic Testing (PGT). This overview aims to raise awareness, especially for couples with the sickle cell trait.
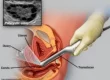
Our clinic now uses the advanced trophectoderm biopsy technique. This method improves upon older practices that biopsied embryos at an earlier stage. We now perform the biopsy on a Day-5 embryo (a blastocyst). This process removes 4-5 cells from the embryo’s outer layer for analysis. Consequently, it offers higher specificity and significantly increases pregnancy success rates. Thus, we want to inform the public about this accessible therapy.
What is IVF? A Foundational Technology
In Vitro Fertilization (IVF) achieves fertilization outside the body. The process involves ovulation induction, egg retrieval, sperm preparation, and embryo culture. Doctors perform egg retrieval and embryo transfer under ultrasound guidance. Strict temperature control is essential for handling sperm and eggs. Without IVF, PGT would not be possible.
What is Preimplantation Genetic Testing (PGT)?
As the name implies, PGT tests for specific genetic defects in an embryo’s DNA before implantation. It involves targeted screening for a known genetic abnormality in the couple. Medical teams first performed PGT in the early 1990s to help couples avoid passing on congenital diseases.
At Medical Art Center, we offer PGT for conditions like sickle cell anemia. We collaborate with the world-renowned Reproductive Genetic Institute of Chicago.
Does PGT Replace Prenatal Testing?
No, it does not. PGT is a pre-pregnancy, research-based test. Prenatal tests like chorionic villus sampling (CVS) or amniocentesis remain the gold standard in obstetrics. Doctors still recommend these tests to confirm the diagnosis if a pregnancy occurs after PGT.
A Brief History of PGT Development
Researchers in the United Kingdom developed human PGT in the mid-1980s. The first unaffected child after PGT for an X-linked disease was born in London in 1989. The technique grew popular in the 1990s for severe disorders like sickle-cell anemia and muscular dystrophy.
In 2013, our team published the first success in Nigeria using PGT to deliver a sickle cell-free baby. PGT is now available for most known genetic mutations, though it remains a relatively new technique in Africa.
Common Reasons for PGT in Nigeria
-
Autosomal Dominant Disorders: One partner carries a defect (e.g., dwarfism).
-
Autosomal Recessive Disorders: Both partners are carriers (e.g., Sickle Cell Disease).
-
X-linked Disorders: One partner carries an X-linked defect (e.g., hemophilia).
-
Chromosomal Abnormalities: One partner carries a structural issue (translocation, inversion).
-
HLA Matching: To select an embryo that is a human leukocyte antigen (HLA) match for a sibling needing a bone marrow transplant, often for sickle-cell disease.
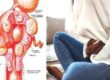
Understanding Sickle Cell Anemia
Sickle cell anemia is a hereditary condition. A mutated form of hemoglobin distorts red blood cells into a crescent shape. The disease causes severe pain, infections, and an increased risk of death. According to the WHO, about 70% of the world’s children with sickle cell disease are born in sub-Saharan Africa. In some parts of Uganda, up to 45% of the population carries the trait.
The Step-by-Step PGT Process for Sickle Cell
1. Preliminary Probe Preparation (8-10 weeks)
Before PGT, the lab must create a specific genetic probe. This requires DNA samples from direct family members (parents or siblings of the couple). The probe will test the biopsied embryo cells.
2. Oocyte (Egg) Retrieval
The female partner receives medication to stimulate multiple egg development. A doctor then retrieves the eggs using a unique ultrasound-guided technique under sedation. The eggs rest in an incubator before fertilization.
3. Fertilization via ICSI
For PGT, fertilization uses Intracytoplasmic Sperm Injection (ICSI). Under a specialized microscope, a single sperm is injected directly into an egg. This ensures high fertilization rates. The embryos then develop in the incubator for 5-6 days to the blastocyst stage.
4. Embryo Biopsy (Trophectoderm Technique)
We perform the biopsy at the blastocyst stage (Day 5 or 6). The embryo then has about 100 cells. Using laser technology, we remove 4-5 cells from the outer layer (trophectoderm), which will form the placenta. This method is less traumatic than older techniques. After biopsy, we immediately freeze all embryos.
5. Embryo Cryopreservation (Freezing)
We preserve biopsied embryos using a rapid-freezing technique called vitrification. We store them in labeled devices within liquid nitrogen tanks, where they remain safely for years.
6. Genetic Analysis in the Lab
The biopsied cells undergo Polymerase Chain Reaction (PCR) to amplify the DNA. Technicians then analyze the DNA to identify the sickle cell status (HbAA, HbAS, or HbSS). We receive results from our collaborating lab within about ten days.
7. Embryo Transfer
We prepare the patient’s uterine lining with medications. Once ready, we thaw the genetically normal (HbAA) embryo(s). Using ultrasound guidance, we transfer one or two embryos into the uterine cavity. A pregnancy test follows two weeks later.
Conclusion and Future Outlook
Today, sickle cell carrier couples in Nigeria can screen their embryos to have a disease-free child. Our clinic has helped many couples achieve this.
While the cost is significant, it offsets a lifetime of medical expenses and stress associated with sickle cell disease. We hope governments and organizations will help make this technique more accessible.
Finally, research continues to improve the speed and reduce the cost of diagnosis. Our experience also shows that environmental toxins and diet significantly impact fertility and IVF success. Therefore, comprehensive detoxification programs, like Mayr therapy, can enhance outcomes by removing heavy metals and allergens from the body.